
Insulet
Acton, MA
Insulet Corporation is a leader in tubeless insulin pump technology. With the aim at returning manufacturing to the US, this medical equipment supplier invested 500 million dollars into a project located on a 25-acre lot in Acton, MA. Avid Engineers was the engineer of record for all project phases including: a 90,000-SF fully automated and validated, cGMP, clean manufacturing build-line, 20,000-SF quality assurance laboratory area, a 195,000-SF facility for senior executives, complete with customer call-center, training/classroom areas, commercial kitchen and cafeteria sized to accommodate over 300 employees, a 35,000-SF warehouse, as well as a 4-level structured parking garage. MEP-FP design features included a 3,000-Ton Chiller plant, cooling towers, fire pump, 13.8-kV medium-voltage distribution, standby generator and UPS. Sustainable design measures coordinated with servicing utility company, Eversource. Measures employed to coincide with eligible incentive programs for mechanical equipment selection, VFD's, lighting and lighting control. Interior lighting COMcheck compliance 52% better than 2015 IECC requirements.

Pfizer
Andover, MA
PFIZER is a global manufacturer of pharmaceuticals, and Andover is home to its, state-of-the-art, R&D facilities and flexible, multi-product manufacturing. Under its Master Service Agreement, Avid Engineers has acted in its role as an in-house engineering consultant, completing a number of projects within PFIZER’s Andover campus. These projects vary from work on administrative office spaces, to lab fit outs, server room improvements, cold room designs, mission critical infrastructure improvements, warehousing spaces and equipment upgrades or replacements. Specific sample projects include: Laboratory space to support the Pfizer ARD Group – program included Sample Drop-Off space F2036, as well as Media Fill Read Room F2036A. Support space for the Pfizer Plasmids Group. An expansion of the existing adjacent Nuclear Magnetic Resonance (NMR) Room to support a second NMR machine. In each, Avid filled the prime consultant role, providing MEP-FP design services, as well as retaining and coordinating all architectural design services.

Millipore-Sigma
Danvers, MA | Bedford, MA | Burlington, MA | Jaffrey, NH
Millipore-Sigma is a global life science company with the goal of accelerating accessibility for better heath for people everywhere. The company designs, tests, and manufactures their medical products while also offering services in the research and drug development fields. For nearly 20 years, Avid has held a master service agree with Millipore-Sigma’s and in that time has completed projects at their headquarters in Burlington, MA as well as in their Danvers MA, Bedford, MA and Jaffrey, NH facilities. Avid’s work has included dozens of projects and hundreds of thousands of square feet of validated, cGMP space including many designs, on several projects, which including incredible office renovations, cleanrooms and infrastructure upgrades. Specific projects that Avid has completed for Millipore-Sigma include numerous ISO-07 and ISO-08 clean rooms and utilities such as process vacuum, health keeping vacuum, compressed nitrogen, dehumidification and humidification, vertical transfer and storage units, gown rooms, pressure differential, cascading pressure arrays, vinyl faced ceiling tiles, and tear drop lighting.

GE Healthcare
Westborough, MA
GE Healthcare Systems is a leading global medical technology and life sciences company. Avid Engineers worked with GE on several Biosafety Level 2 projects over multiple projects and locations. Biosafety Level 2 or BSL-2 laboratory spaces are used to study moderate-risk infectious agents or toxins. BSL-2 design requirements include hand washing sinks, eye washing stations, and doors that close and lock automatically. BSL-2 laboratories must also have access to equipment that can decontaminate laboratory waste, including an incinerator, an autoclave, or another method of decontamination. Avid’s design was created to include or accommodate all of those requirements, as well as multiple infrastructure upgrades related to lab support utilities.

Edesia Global
Kingston, RI
Edesia Global Nutrition is a non-profit social enterprise making life-saving foods that help solve the world’s malnutrition health crisis. Edesia works to create milk powder-based food formulas to feed and treat malnourished children. Avid Engineers was responsible for designing the MEP-FP systems for Edesia’s 85,000-SF processing and storage facility on a green grass site in North Smithfield, RI. The facility was required to be compliant with both the European Commission Enterprise’s Rules for Governing Sterile Medicinal Products and the FDA’s cGMP Practices as described in Title 21 for Food and Drugs. The space included 10,000-SF of office space, 25,000-SF of preparation space and 50,000-SF of warehouse. Avid Engineers provided a central make-up air system that provided outdoor air to all air handling units. The air handling units that provided air for the clean manufacturing areas were required to meet ISO-08 room conditions by maintaining particle count and set air change rate; the air handling units were paired with HEPA filtered ceiling diffusers and the space set to maintain positive pressure.

Freudenberg Medical
Beverly, MA
Freudenberg Medical is part of the Freudenberg Group. Their services range from the design and manufacture of minimally invasive, catheter, and handheld technology to the development and production of medical components utilizing advanced materials and processes. Avid Engineers was responsible the MEP-FP design of Freudenberg’s 36,500-SF ground up, manufacturing facility in Beverly, MA. These systems were designed around Freudenberg’s ISO-08 requirement for their manufacturing space. The air handling units that provided air for the clean manufacturing areas were required to meet ISO-08 room conditions by maintaining particle count and set air change rate; the air handling units were paired with HEPA filtered ceiling diffusers and the space set to maintain positive pressure.
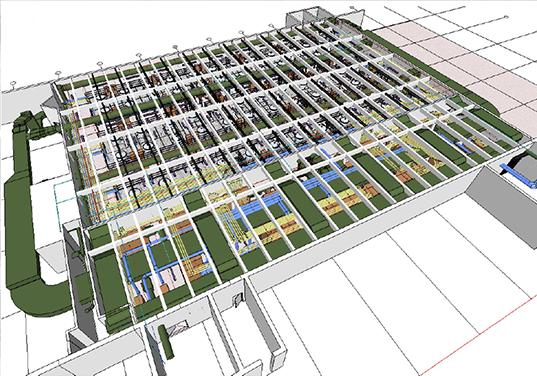
Lantheus Medical Imaging
Billerica, MA
Lantheus Medical Imagining is a global leader in the field of medical imaging. Formerly Bristol-Myers Squibb, Lantheus develops, manufactures and commercializes essential diagnostic imaging agents and products that help healthcare professionals identify disease and improve patient treatment and care. Avid Engineers has designed a series of projects at Lantheus Medical Imaging’s location in Billerica, MA. Projects include research and development laboratories, locker rooms, transport loading docks and storage areas – all designed per guidelines required to facilitate safe use of radioactive isotopes used in medicine. The effort included the adaptive reuse of existing equipment to support the new design, and took careful steps to not disturb any of the adjacent laboratory spaces or processes.

Addgene
Cambridge, MA
Addgene is a non-profit plasmid repository in the life science center of Kendall Square in Cambridge MA. Addgene facilitates the exchange of genetic material between laboratories by offering plasmids and their associated cloning data to not-for-profit laboratories around the world. In 2015, Avid Engineers was responsible for the design of the mechanical, electrical, plumbing and fire protections systems for Addgene’s facility at 75 Sidney Street in Cambridge, MA. The project included the renovation and expansion of existing storage space into a new Biosafety Level 2 laboratory space, as well as the distribution of process gases, vacuum and fume hoods while facilitating the adaptive reuse of any existing equipment in the existing building space in order to create their final design.






